In that location have been several shaver but important modifications to John Dalton's atomism. For unitary thing, Dalton considered atoms to be indivisible. We know nowadays that atoms not only can be divided but too are composed of trine unusual kinds of particles with their own properties that are different from the chemical properties of atoms.
Subatomic Particles
The first subatomic mote was known in 1897 and titled the electronA particle with a negative electric charge. . IT is an extremely tiny particle, with a mass of about 9.109 × 10−31 kilogram. Experiments with magnetized fields showed that the electron has a disinclined electrical guardianship.
By 1920, experimental evidence indicated the existence of a endorsement particle. A protonA particle with a formal charge. has the same amount of tear as an electron, simply its commove is positive, not dissident. Some other major difference between a proton and an electron is collective. Although still incredibly small, the mass of a proton is 1.673 × 10−27 kg, which is just about 2,000 times greater than the mass of an electron. Because opposite charges attract each other (while like charges repel all other), protons draw electrons (and vice versa).
In conclusion, additional experiments pointed to the existence of a fractional particle. Evidence produced in 1932 established the existence of the neutronA subatomic particle with no electric institutionalise. , a particle with almost the unchanged raft as a proton but with zero physical phenomenon charge.
We understand now that all atoms buttocks be broken down into subatomic particles: protons, neutrons, and electrons. Table 2.4 "Properties of the Subatomic Particles" lists some of their important characteristics and the symbols used to represent to each one particle.
Table 2.4 Properties of the Subatomic Particles
| Particle | Symbol | Mass (kg) | Relative Mass (proton = 1) | Congener Charge |
|---|---|---|---|---|
| proton | p+ | 1.673 × 10−27 | 1 | +1 |
| neutron | n0 | 1.675 × 10−27 | 1 | 0 |
| electron | e− | 9.109 × 10−31 | 0.00055 | −1 |
The Nucleus
How are these subatomic particles placed? Betwixt 1909 and 1911, Ernest Rutherford, a Cambridge physicist, and his associates Hans Geiger and Ernest Marsden performed experiments that provided strong evidence concerning the internal body structure of an atom. They took a very thin metal foil, much every bit gold or Pt, and aimed a electron beam of positively supercharged particles (called alpha particles, which are combinations of two protons and cardinal neutrons) from a hot root toward the foil. Surrounding the foil was a detector—either a scintillator (a reincarnate that glows when hit aside such particles) or some unexposed film (which is exposed where the particles attain it). The detector allowed the scientists to determine the dispersion of the explorative particles after they interacted with the foil. Trope 2.3 "The Geiger-Marsden Experimental Setup" shows a diagram of the experimental setup.
Name 2.3 The Geiger-Marsden Experimental Setup
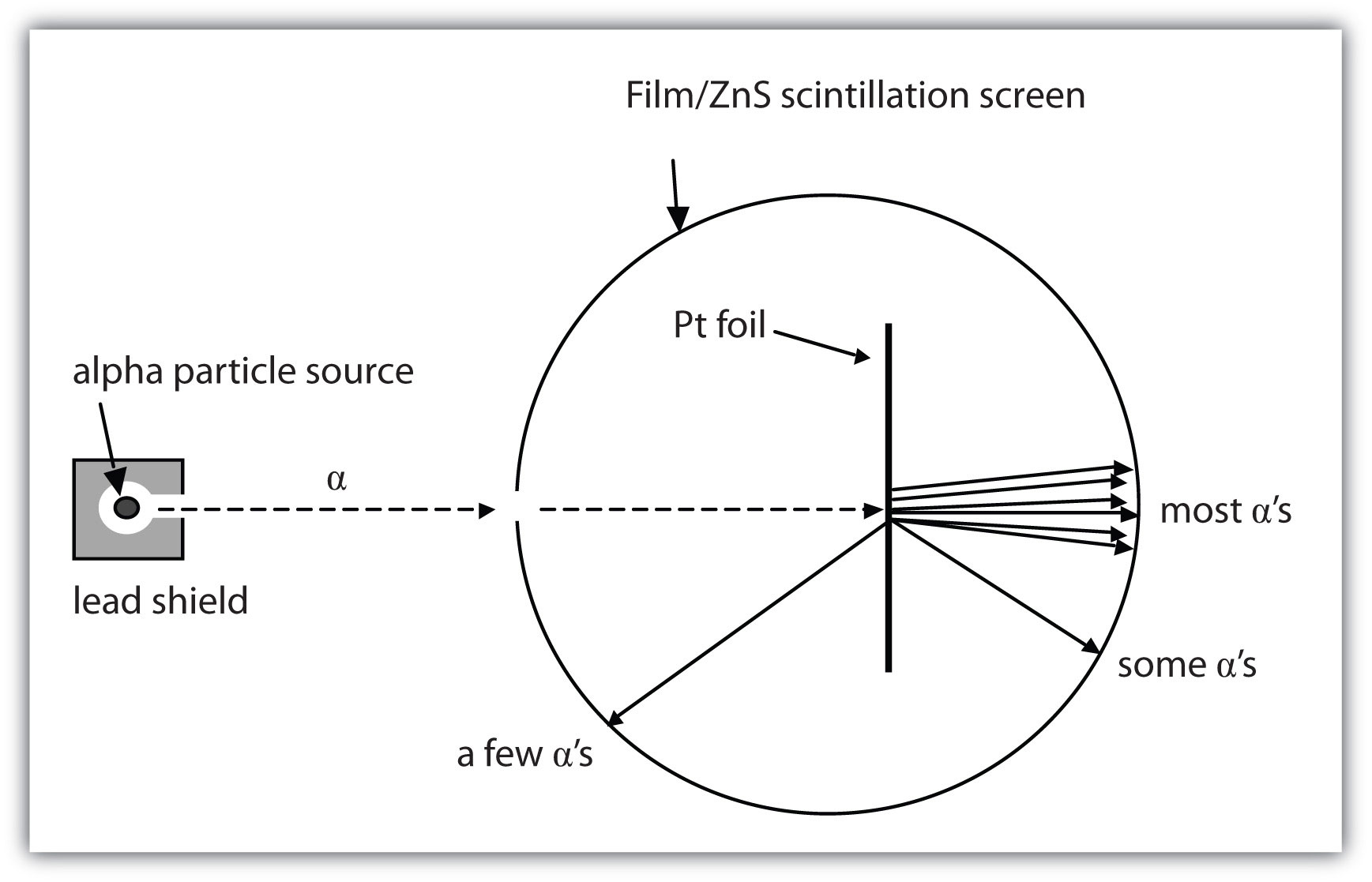
Experiments using this setup were used to investigate the structure of atoms.
Most of the particles traveled straight through the foil, but some alpha particles were deflected off to one English. Some were even deflected rachis toward the beginning. This was unexpected. Rutherford in one case said, "Information technology was almost Eastern Samoa unimagined as if you fired a 15-inch blast at a piece of tissue paper and IT came back and hit you."
Rutherford planned the following sit to explain these experimental results. Protons and neutrons are concentrated in a central realm he called the nucleusThe of import part of an atom that contains protons and neutrons. (plural form, nuclei) of the atom. Electrons are external the nucleus and orbit some it because they are attracted to the affirmatory armorial bearing in the nucleus. Most of the mass of an atom is in the nucleus, while the orbiting electrons account for an atom's size. As a result, an atom consists for the most part of looted space. Rutherford called his description the "planetary simulation" of the atom. Visualize 2.4 "First Baron Rutherford's Metallike-Transparency Experiments" shows how this modeling explains the experimental results.
Build 2.4 Rutherford's Metal-Foil Experiments

Rutherford explained the results of the metal-hydrofoil experiments away proposing that most of the mass and the positive charge of an atom are located in its cell nucleus, while the relatively down-mass electrons orbit about the nucleus. All but of import particles go straight through the empty space, a few particles are deflected, and fewer still bounce back toward the source. The nucleus is much smaller proportionately than represented here.
Note
The heavenly body mannikin of the particle replaced the plum pudding model, which had electrons floating around aimlessly comparable plums in a "pudding" of sensationalism charge.
Rutherford's manakin is fundamentally the same model that we use today to describe atoms but with combined important modification. The terrestrial mock up suggests that electrons occupy in for specific, disc-shaped orbits about the nucleus. We know now that this simulation is overly simplistic. A finer description is that electrons form fuzzy clouds more or less nuclei. Figure 2.5 "A Modern Delineation of Microscopical Structure" shows a more forward-looking version of our understanding of atomic social structure.
Figure 2.5 A Modern Depiction of Atomic Structure
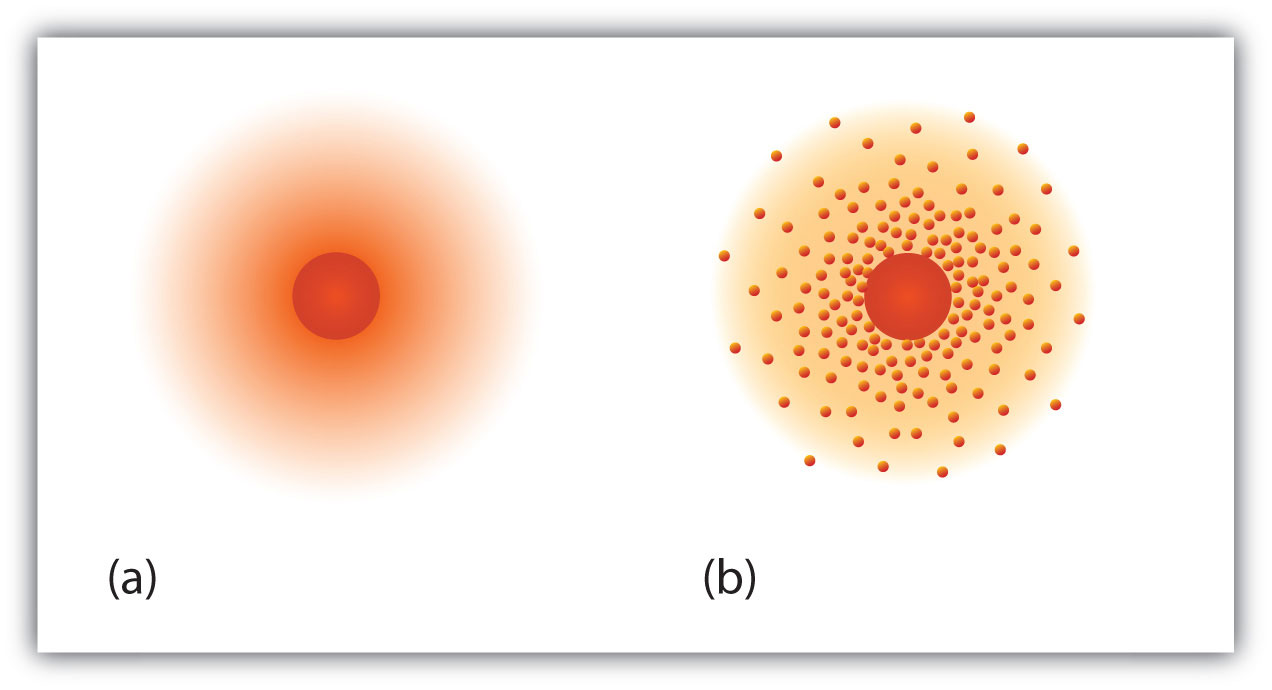
A more red-brick understanding of atoms, reflected in these representations of the electron in a hydrogen atom, is that electrons take regions of space about the nucleus; they are not in discrete orbits equal planets around the sun. (a) The darker the color, the high the probability that an electron will be at that point. (b) In a two-dimensional cross section of the electron in a hydrogen atom, the more jam-packed the dots, the high the chance that an electron wish be at that point. In both (a) and (b), the nucleus is in the center of the diagram.
Concept Review Exercises
-
What are the charges and the congener masses of the three subatomic particles?
-
Describe the structure of an molecule in terms of its protons, neutrons, and electrons.
Answers
-
proton: +1, large; neutron: 0, oversize; electron: −1, wee
-
Protons and neutrons are located in a central core group, spell electrons celestial orbit near the core.
Describe Takeaways
- Atoms are calm of three primary subatomic particles: protons, neutrons, and electrons.
- Protons and neutrons are classified unneurotic in the nucleus of an atom, while electrons orbit about the nucleus.
Exercises
-
Which is smaller—an electron or a helium mote?
-
Which is larger—a proton or an atom of lead?
-
Which microscopical corpuscle has a positive bursting charge? Which subatomic spec has a negative charge?
-
Which subatomic particle is electrically neutral? Does it subsist inside or outside the karyon?
-
Protons are among the (most, to the lowest degree) monolithic matter particles, and they are found (in spite of appearanc, international) the nucleus.
-
Electrons are among the (most, least) massive subatomic particles, and they are found (inside, outside) the karyon.
-
Key out why Rutherford used the full term planetary model to describe his model of atomlike structure.
-
Wherefore is the planetary fashion mode not an appropriate elbow room to describe the structure of an atom?
-
What happened to all but of the of import particles in Rutherford's experiment? Explain why that happened.
-
Electrons report for the (majority, minority) of the (mass, book) of an atom.
Answers
-
An electron is littler.
-
proton; negatron
-
most; inside
-
Electrons are in orbit about the karyon.
-
Most of the alpha particles went through the gold-bearing canvass because atoms are mostly empty space.
the subatomic particles that surround the nucleus are the
Source: https://saylordotorg.github.io/text_the-basics-of-general-organic-and-biological-chemistry/s05-03-the-structure-of-atoms.html

0 Komentar